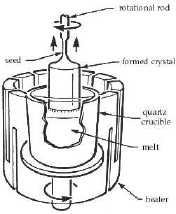
Figure 1: Czochralski process For growing doped crystal, impurities can be added to the melt. Some of the most common impurities are P, As, Sb, B, and Al. One problem encountered in growing doped crystal is the difference in impurity concentration of dopant in the solid and the liquid state. The ratio of impurity concentration in solid, Cs, to the concentration in liquid, Cl, is called the segregation coefficient. The pulling rate of the seed crystal is limited to the concentration gradient of the growing crystal. Care must be taken that the dopant concentration does not fluctuate too much along the direction of crystal growth. The presence of oxygen in silicon also poses another problem. The oxygen usually comes from the erosion of the quartz crucible. The concentration of oxygen found in silicon depends on the rotating rate. Fast rotations tend to produce higher concentration of oxygen atoms. Although fast growth rate (faster rotation of the seed crystal out of melt) helps dislocations propagate out of crystal, it also introduces large amount of unwanted oxygen atoms. Most of the oxygen ends up as SiO2. They tend to segregate along dislocations. Circuits built on that part of the crystal are therefore defective. Hence controlling the rotational rate and keeping oxygen concentration level low are crucial to crystal growth.