The order Hymenoptera as a whole has long been known to possess several unusual features intriguing and puzzling to biologists. The most obvious of these is the evolution of social behavior, in which most members of a community sacrifice their own reproductive potential, to provide food and protection for the few reproductive members and their offspring. It is clear from the presence of such unusual life histories that some Hymenopterans have been subject to strong divergent selective pressures which have effected various aspects of their biology. It is perhaps no great surprise, then, to find a great deal of variation in the systems of sex determination.
Arrhenotokous haplodiploid sex determination -- in which haploid eggs develop as males, and diploid individuals are female -- is the rule in the order. However, increasing evidence suggests that exceptions to this rule are common, or even ubiquitous. Diploid males exist in many populations in disparate species (Cook, 1993a and references therein). Many species also harbor intracellular bacteria, and at least one species has been shown to possess a "selfish" supernumerary chromosome. Both of these manipulate the sex determination scheme to increase their own inheritance to greater than Mendelian rates. Thelenotokous species also exist, in which females reproduce parthenogenically to produce diploid female offspring.
All these unusual characteristics in the Hymenoptera makes it tempting to dismiss the Hymenoptera as an evolutionary aberration, victims of runaway selection. It would be premature, however, to say that mechanisms of sex determination bear no common ground within the order, or even with other orders and phyla. It is true that haplodiploid sex determination is rare among animals, and restricted to a few groups in arthropods. It is also true that no model proposed to date is sufficient to explain sex determination in more than a subset of Hymenoptera. However, no molecular studies of the genes involved in sexual determination have been done. It is possible, therefore, that some of the underlying mechanisms are in fact conserved between Hymenopteran groups, and within larger taxa. Evidence for or against such a hypothesis, however, awaits the collection of molecular data.
Regardless of such homologies, it seems clear that the sex determination systems of the Hymenoptera reflect in many ways the peculiar life histories and conditions of individual species. In the space that follows, we discuss these peculiarities and the models which explain them to one degree or another. Lastly, we discuss briefly the relation between these two, which may with further research help bring order to their complexity.
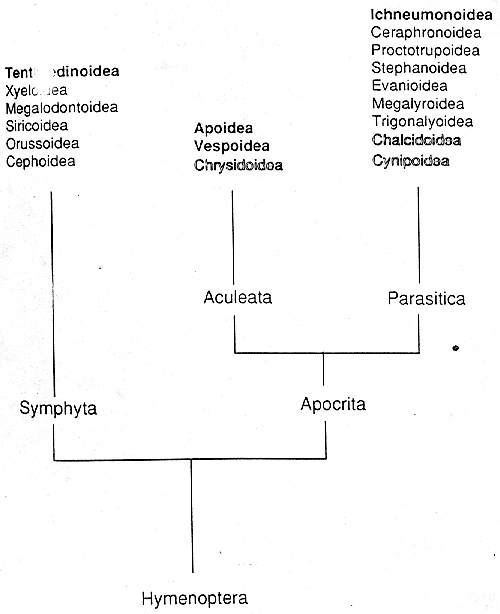
Hymenopterans follow a great many life histories. The superfamily Aculeata (Figure 1), which includes the bees, ants, and vespid wasps, includes all of the social Hymenoptera, as well as many solitary species. Sociality, in which some members of a community forego reproduction to care for offspring produced by another individual, presents unique reproductive challenges that are closely intertwined with sex determination. The superfamily Parasitica contains many families of parasitoid wasps. Parasitoids, which coevolve extensively with their host species, face special reproductive constraints due to the demands of locating hosts. Such constraints may in part explain the difficulty in finding a unifying model for Hymenopteran sex determination (see below). The sawflies, or Symphyta, are a little known infraorder group of herbivorous and carnivorous Hymenoptera that is exclusively solitary.

Figure 1: Phylogeny of Hymenoptera superfamilies. Symphyta are sawflies, Aculeata are bees, ants, and vespid wasps, and Parasitica are parasitoid wasps. (From Cook, 1993a).
Facultative Control of Sex Ratios
The ability of females in arrhenotokous lineages to determine the sex of their offspring has important ecological and evolutionary implications for both Parasitica and social Aculeata, and may act as a selective pressure for certain forms of sex determination. Facultative sex allocation allows parasitoid wasps to fit their offspring to the quality of host they are provided. Social bees, wasps, and ants can modify sex ratios within colonies to maximize relatedness among members, and to generate a workforce appropriate to surrounding conditions.
In some parasitoid wasps that prey on diffusely distributed hosts and normally inbreed, a situation known as local mate competition may skew sex ratios (Hardy, 1994). Many eggs are lain by one or a few females on a single host. Sometimes adults only mate immediately after emergence with males emerging from the same host. Mothers can produce more females under these conditions, generating only sufficient numbers of males to fertilize the females. The skewed sex ratio generates the largest number of offspring capable of passing their genes on to future generations (Godfray, 1994).
Sex ratios can also be adjusted to control for the quality of the host (Hardy, 1994; Godfrey, 1994). Males are less adversely affected by being undernourished, since they die soon after mating. Females, on the other hand, need more sustenance to produce viable eggs. Consequently, it is advantageous to give females higher quality hosts as larval food. In species that lay one egg per host, females can be given larger hosts. In species with multiple eggs on a host, this can be done by increasing the female to male ratio as the host size increases. Such control of sex ratio, besides being common in Parasitica, has also been shown in herbivorous Symphyta (Craig, et al., 1992). It is important that the sex determination system not be single locus sex determination (to be discussed below) in these species: infertile diploid males would regularly be produced due to increasing homozygosity.
In social Hymenoptera, control of sex ratios allows for the overproduction of female workers, and limited production of reproductive males (and females, as we will discuss briefly below). Due to control over the sex ratio, males are only produced when mating occurs, while workers are produced throughout the year. In eusocial species (species that are social year round), the control of the sex ratio is not determined entirely by the queen, but can be altered by the workers. By Hamilton's model of inclusive fitness (1964), full sisters share 3/4 of their genome with their sisters, but only 1/2 with their mother and 1/4 with their brothers. The mother, on the other hand, shares 1/2 of her genome with both sons and daughters. Consequently, there is a conflict between workers and queens over the desired production of each sex (reviewed in Crozier and Pamilo, 1996).
Despite the conflicts over sex allocation, the differential
relatedness of sisters is presumably one of the factors that has made
sociality widespread among the Hymenoptera (Crozier and Pamilo,
1996). Hamilton's model (1964) predicts that individuals will only
forego reproducing when they can get more of their genes into
following generations by helping a relative reproduce. The efforts of
many sisters working together to help their mother or sister
reproduce can generate more offspring than their solitary efforts.
Hamilton argues that Hymenopteran workers are exclusively female
because their greater relatedness increases their interest in their
sisters' reproductive success relative to their less-related
brothers.
Control over sex ratio allows some species, especially halcitine
bees, to be facultatively social. These species are not eusocial: for
part of the year, the colony consists of a single solitary individual
(Michener, 1974). But, for the rest of the year, related females
remain in the nest with each other and divide labor among themselves.
Included in this division of labor is a division of reproductive
activity, with one, or a few, individuals generating all of the
offspring. In temperate climates, Hymenoptera only have sufficient
time to produce workers and reproductive individuals if the season is
long enough. If sex ratio is under their control, they can adjust the
sex ratio of their offspring to generate a social structure to fit
their climate (Packer, et. al., 1989; Eickwort, et. al, 1996). In
extreme places, like high latitudes or elevations, they can do away
with workers, and become solitary (Packer , at. al. 1989; Eickwort,
et. al, 1996). In less forbidding places they can generate workers,
who allow for the production of more reproductive offspring. Debate
remains whether this ability is due to developmental plasticity or
evolution, but current evidence suggests it is due to developmental
plasticity (Eickwort, 1996).
[Go here for more on the developmental plasticity of workers]
Selfish genes, selfish microbes
In addition to unique selective pressures governing sex determination in some Hymenoptera, analyses of the evolution of sex determination in the order are hindered by the fact that in many cases, the actual Hymenopteran being studied is not the only organism with a vested interest in determining its sex. This rather surprising condition is a result of intracellular bacteria of the genus Wolbachia. These bacteria are widespread in insects, having been found in 16 percent of Hymenopteran species surveyed. They may be even more prevalent in social Hymenoptera -- a recent survey found the bacteria present in half of all ant species tested (Wenseleers et al, 1998 and references therein). Since Wolbachia is cytoplasmically inherited, its fitness is dependent on female production. Thus, infection tends to produce a female-biased sex ratio.
The mechanism for this effect takes two forms. One involves the loss of the paternal genome in a mating between infected males and uninfected females (reviewed in Hoffmann and Turelli, 1997). This results in embryonic death in diploid species, and male development in haplodiploid species like Hymenoptera. Thus, an uninfected female is prevented from producing any daughters. Another mechanism is the induction of parthenogenic behavior. The duplicated chromosomes of an infected, unfertilized egg fail to separate during the first mitotic cycle, producing a diploid female individual (reviewed in Stouthamer, 1997). Both of these mechanisms increase the likelihood that the bacteria will be more prevalent with each subsequent generation. They do not necessarily maximize the fitness of their hosts, however. In fact, many parthenogenic species harboring Wolbachia return to sexual reproduction upon antibiotic elimination of the parasite (Stouthamer, et al, 1990a).
A second peculiarity in Hymenopteran sex determination is the existence, in at least one species, of a so-called selfish chromosome which is inherited at greater than Mendelian proportion. When a female mates with a male carrying this chromosome, called the Paternal Sex Ratio or PSR chromosome, it produces only male progeny, all of which will then pass on the PSR chromosome when they mate. Thus, while females produced by a normal fertilization pass on the paternal genome to only half their offspring, males produced by matings of PSR-males give their PSR chromosome to all their offspring.
The PSR chromosome is a small supernumerary chromosome with no known beneficial functions. It consists mostly or entirely of heterochromatin, organized in blocks of repeating sequences (Beukeboom and Werren, 1993). According to deleltion analyses by Beukeboom and Werren (1993), these repeats vary from not required to essential for PSR function and transmission. In eggs containing PSR, the rest of the paternal genome forms a dense mass prior to the first metaphase. It replicates once prior to the first division, but not subsequently, and is eventually lost (Reed and Werren, 1995). The result is a haploid male with only the PSR chromosome remaining from its paternal genome. The existence of a "genomic parasite" such as this naturally raises questions about its evolution and mechanism for action. Although research is continuing, little has been discoved to date.
The models
Complementary Allele Sex Determination
One model for sex determination in Hymenoptera is the complementary sex determination (CSD) or allelic diversity model, first proposed by Whiting (1943) early in this century. Evidence for it has accumulated in several different Hymenopteran lineages. In this model, sex is determined by the complementarity of alleles at a certain locus. If the alleles are different, a female develops. If the locus is hemizygous or homozygous, a male develops. The primary evidence for this model comes from inbreeding experiments. Diploid Hymenopteran males are rare in nature, and are usually infertile. With forced inbreeding, they have been generated in the laboratory in a number of species. Molecular evidence to support this theory has not yet been uncovered, but may emerge in the next few years.
The earliest evidence of CSD came from a set of breeding experiments done by Whiting (1943) on Bracon hebetor (previously Habrabracon juglandis). In his previous work, Whiting had found many morphological traits with classical Mendelian patterns of inheritance, from which he constructed a linkage map. When he backcrossed male offspring to their mothers, he found males that must posses two copies of some traits, indicating diploidy. Whiting calculated that in the population he was working with, at least nine alleles existed at the sex determining locus.
Whiting's work has spawned a series of similar breeding experiments. Periquet, et. al. (1993) bred Diadromis pulchellus, an endoparasite of a Lepidopteran, in captivity. Through a series of planned crosses they generated diploid males, and compared their resulting sex ratios to the sex ratios predicted by Mendelian genetics for single and double locus sex determination models. They detected diploid males in two ways: coat color and allozyme variation. Normal bees are black, but they were able to raise less fit recessive yellow mutants in the laboratory. By mating wildtype females to yellow males, and then mating the heterozygous female offspring back to their fathers, they produced homozygous yellow females. These females were mated to black males. Any black males resulting from the cross must be diploid, due to the dominant color gene from their dark coated father. In another method, two enzymatic loci with multiple variant alleles that can be detected by gel electrophoresis. Inbred females were outcrossed to unrelated inbred males, and both parents analyzed postmortem for heterozygosity at the enzymatic loci. If the mother had any different alleles than the father, they analyzed male offspring for heterozygosity at the same locus. From these crosses, they found that males were generated in numbers consistent with a single locus multiple allele model of sex determination.
Inbreeding experiments like these have been repeated in a number of Hymenoptera species. Currently, the single locus CSD model may account for the diploid males observed in between 8 and 33 species (Cook, 1993a). These species span the range of the order Hymenoptera, from Symphyta to Parasitica to Aculeata. This line of research has several shortcomings. By using inbred lineages, it is possible that homozygosity is developed at other loci that are involved in sex determination, masking their influence. In these cases, multiple loci multiple-allele sex determination would look like single locus sex determination. Also, it seems possible that inbreeding would lead to homozygosity for some recessive loss-of-function mutations farther downstream in the sexual development cascade. Such mutations might result in an increase in diploid males as well. Lastly, the results of these experiments also do not completely preclude other methods of sexual determination, which are discussed below.
No work has been done to clarify the molecular details of these pathways. Crozier (1971) speculated that the sex determining locus (or loci) may produce proteins that are only active as heteropolymers. Why exactly any heteropolymer would be sufficient to produce activity while any homopolymer would be inactive remains unclear. There have been calls in the literature for molecular work to make sense of the model (Beukeboom, 1995), but the work has been slow to emerge.
The first steps of molecular research have been done on the model Hymenopteran, Apis mellifera. Previous studies, akin to those done by Periquet, et. al. (1993), established that the honey bee uses single locus CSD like Bracon hebetor (Cook, 1993a). Hunt and Page (1995) expanded on this work by generating a linkage map of the most of the honeybee genome with RAPD markers (Randomly Amplified Polymorphic DNA). Using the map, they established the chromosomal relationship of the sex determining locus with other genetic markers. In this work they used 10 nucleotide long random primers to amplify random sections of the honeybee genome. They selected primers for further study that generated DNA fragments that were polymorphic in length between the parents of the laboratory colony they used as a source of bees. With these fragments, they could compare the genotypes of male (and usually haploid) offspring. They generated a linkage map for the honeybee genome by comparing the inheritance pattern of allozyme markers that varied in the mother and that could be assayed for with protein gel electrophoresis with the RAPD marker patterns. This information, which locates loci near the sex determining locus, sheds little light on sex determination, but may be useful for future studies.
In the absence of much molecular work, ecologically oriented studies have been done to examine the effects of single locus sex determination on other Hymenoptera. Ross, et. al. (1993) studied difference in the rate of occurrence of diploid males in two populations of Solenopsis invicta, the South American fire ant. In the 1930's the fire ant was introduced into Mobile, Alabama, and has spread across much of the southern US. Ross's lab had previously performed breeding experiments to show that the fire ant has a complementary sex determination system, and that diploid males are common in the North American populations (Ross and Fletcher, 1986).
By comparing the rate at which diploidy occurs in nests in North and South America, Ross et. al (1993) could demonstrate that the fire ant has gone through a significant bottleneck in North America in historical times due to a founder effect, and is less genetically diverse than the population from which it originated. Diploidy was measured by doing Western blots for housekeeping proteins that had been found to be polymorphic. Heterozygosity at any of the enzymatic loci is a sign of diploidy. With this measure, they showed that the Argentinean populations have a lower rate of male diploidy.
A possible alternative to or extension of single locus complementary sex determination is multiple locus CSD. In this model, proposed by Crozier (1971), heterozygosity at any of several sex determining loci results in females. Crozier (1971) proposed that it might account for all Hymenopteran sex determination. There is currently evidence against it in some species (eg, Cook, 1993b), and no solid proof in any species. Yet, it is not a completely inviable theory. In consistently inbreeding species such as parasitoid wasps, it is less likely to lead to frequent diploid male production than a single locus system. Since a multiple locus model can collapse into a single locus model when homozygosity develops in a population at all but one of the loci, it may account for the evidence for single locus CSD. Since many of the populations used for single locus CSD are inbred laboratory stocks, this is a possibility. Yet, as there is very little data to explain how single locus CSD works on a molecular level, conceptualizing how multiple loci CSD works is difficult.
Genic balance
According to this model, male-producing loci have a non-cumulative effect, whereas female-producing loci are cumulative. Thus, in haploid individuals, the female-producing stimulus is twice as weak as in diploid individuals. The male producing stimulus, in contrast, is constant regardless of ploidy. The result is that male-producing stimuli dominate in haploid individuals, while female producing stimuli dominate in diploid and polyploid individuals. This model was first developed by Cunha and Kerr (1957), when diploid males were only known in one genus. Increasing evidence of the existence of diploid males throughout the Hymenoptera (see Cook, 1993a and references therein) has prompted the more recent view that it is invalid (Cook, 1993a). Efforts to prove it have centered on a search for partially feminized characteristics in diploid males, with the idea that residual effects of female-inducing genes would manifest themselves in morphological differences between haploid and diploid males. The results of such studies have been contradictory, although more recent studies suggest that diploid males in fact possess larger structures than their haploid counterparts; this may in fact be largely due to their larger genome (see Cook, 1993a and references therein). In any case, the genic balance model requires the independent evolution of modifications many times during Hymenopteran evolution in order to account for diploid males.
Maternal - Zygotic balance
This model, though not new, is untested. It was first proposed by Crozier (1977) and further developed recently by Cook (1993b). Cook argues that in species where inbreeding is frequent, the CSD model is unlikely to account for sex determination, since diploid males -- which are sterile -- would be produced at relatively high frequency. Thus he suggests that a situation similar to that in Drosophila might exist. In this system, a maternally transcribed factor, perhaps similar to Drosophila daughterless, would stimulate male development. Zygotic genes, perhaps similar to Drosophila sis would compete, stimulating female development. A diploid compliment would successfully inhibit male development and produce a female, while a haploid compliment would result in males. Such a model obviously has precedent in other insects, and support for its existence in Hymenoptera would likely come from molecular studies which hopefully are forthcoming. Unfortunately, this model cannot account for widespread diploid males any more easily than the genic balance model above can.
Genomic Imprinting
The genomic imprinting model suggests that it is not haploidy or diploidy per se which determines sex, but the presence or absence of a paternally-imprinted chromosome compliment early in development. At some point during male development, a portion of the male germline is modified, or imprinted. All eggs, regardless of ploidy, which possess only maternally derived chromosomes, would develop as males. Any egg receiving a full paternal genome, on the other hand, would develop as female due to the paternal imprint.
The only explicit test of this model was conducted recently in the parasitic wasp Nasonia vitripennis, the same species which also possesses the "selfish" PSR chromosome discussed above. Dobson and Tanouye (1998) took advantage of this chromosome, utilizing a triploid female strain of the wasp and crossing it with males harboring the PSR chromosome as shown in Figure 2. According to most other models, a diploid, fertilized egg ought to develop as a female. But if chromosomal imprinting occurs as proposed above, then a chromosome compliment of paternal origin is necessary for male development. Thus, the above mating would produce no females, just as a mating with diploid females would not. Instead, diploid eggs which lost their paternal genome via the action of the PSR chromosome would in fact develop as sterile males. This is in fact what Dobson and Tanouye observed.
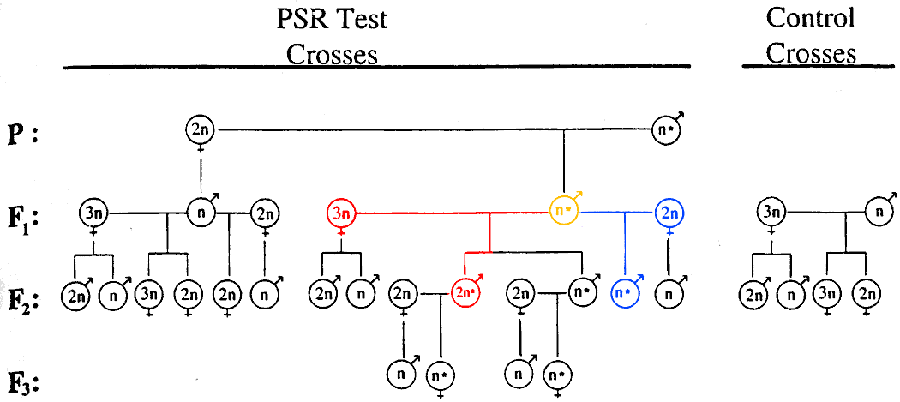
Figure 2. The crosses performed by Dobson and Tanouye (1998) to support the genetic imprinting model. Crosses between a PSR-carrying male (yellow) and a triploid female lead to only male progeny (red), even in cases where the individual produced is diploid. The corresponding control crosses with a diploid female (blue) and between non-PSR indivduals (right) are shown as well. The results of these crosses are consistent only the genomic imprinting model. From Dobson and Tanouye, 1998 (color added).
As they mention, there are a few other possible interpretations of their data. It does not exclude the interpretation that in fact, the triploid females were homozygous at some sex-determining locus or loci, and, as in the complimentary sex determination model discussed above, the diploid males developed as a result of this homozygosity. This interpretation was rendered unlikely by work done by Skinner and Werren (1980), who failed despite repeated inbreeding to generate diploid males in N. vitripennis. Furthermore, Werren (1997) also found that Wolbachia bacteria cause segregation failure in haploid, unfertilized eggs, causing them to develop as homozygous diploid females as described above. While such research effectively disproves the CSD model, it also suggests that the mechanism for Wolbachia-mediated genomic duplication may be complicated. If the genomic imprinting model is correct, then Wolbachia must, in addition to inducing segregation failure, somehow bypass or emulate the paternal imprinting apparatus. Given the widespread nature of Wolbachia in arthropods, and the varied effects attributed to it as discussed above, it seems possible the bacteria may be involved in this process (Werren, 1997, Dobson and Tanouye, 1998).
A final possible interpretation of the data is that the PSR chromosome itself contains a master gene responsible for triggering male development independent of the loss of the paternal genome. As the authors point out, however, deletion analyses of the PSR chromosome have failed to produce any forms of the chromosome that induce male development without loss of the paternal genome (Beukeboom and Werren, 1993). Such a gene would thus have to be closely linked to the portions of the chromosome responsible for genome loss.
Although Dobson and Tanouye's work strongly suggests the importance of paternal imprinting in N. vitripennis, it is unclear whether the model is more widespread. Some studies have documented the existence of thelytokous populations in other species. In these populations, diploid, female individuals develop from unfertilized, haploid eggs via a mechanism which, unlike that mediated by Wolbachia, cannot be eliminated by antibiotic or high-temperature treatment (Stouthamer et al, 1990a). Clearly, paternally-restricted imprinting is not likely to exist in such species.
The elusive unified model for sex determination
While in recent years evidence has mounted that a great number of biological pathways are conserved across the animal kingdom, one developmental process which for the most part has evaded this grand display of commonality is that of sex determination, which in contrast seems to have evolved rather rapidly (Marín and Baker, 1998). Hymenoptera seems perhaps to provide an extreme example of this.
The models discussed above each have strengths and weaknesses. But none conveniently explains sex determination in all Hymenopteran species studied. Single locus sex determination is the oldest model, but it can not explain sex determination in all Hymenopterans. Other models, such as maternal-zygotic effects and genomic imprinting, may be appropriate for at least some Hymenoptera. Wolbachia bacteria, commonly found in many insects, may also be found to have an important role in sex determination in many Hymenoptera species.
Sex determination is closely intertwined with the life histories of Hymenoptera. Perhaps by looking to the unique life histories of various Hymenopteran taxa, it will become easier to understand how so many different models may have evolved. The unique selective pressures on sex determination mechanisms were probably quite important in producing the great differences with other taxa. Hamilton proposed as much by linking haplodiploidy to social behavior in his model of inclusive fitness in 1964. But evolutionary differences are most easily understood by looking in tandem at the similarities. These similarities, however, are likely only to be found by molecular research into the mechanisms of sex determination. Only armed with such data will we be able to begin understanding the evolution of this unique order.
Beukeboom, L., 1995. Sex determination in Hymenoptera: a need for genetic and molecular studies. Bioessays. 17: 813-817.
Beukeboom, L.W. and Werren, J.H., 1993. Deletion analysis of the selfish B chromosome, paternal sex ratio (PSR), in the parasitic wasp Nasonia vitripennis. Genetics 133: 637-648.
Cook, J. 1993a Sex determination in hymenoptera: a review of models and evidence. Heredity. 71: 421-435.
Cook, J. 1993b. Experimental tests of sex determination in Goniozus nepjantidis (Hymenoptera: Bethylidae). Heredity. 71: 130-137.
Craig, T., et. al. 1992. Facultative sex ratio shifts by a herbivorous insect in response to variation in host plant quality. Oecologia. 92: 153-161.
Crozier, R. H. 1971. Heterozygosity and sex determination in haplo-diploidy. Am. Naturalist. 105: 399-411.
Crozier, R.H. 1977. Evolutionary genetics of the Hymenoptera. Ann. Rev. Entomol. 22: 263-288.
Crozier, R., and Pamilo, P., 1996. Evolution of social insect colonies: sex allocation and kin selection. Oxford, England: Oxford University Press.
Cunha, A.B. and Kerr, W.E. 1957. A genetical theory to explain sex determination in arrhenotokous parthenogenesis. Forma et Functio. 1: 33-36.
Dobson, S.L. and Tanouye, M.A. 1998. Evidence for a genomic imprinting sex determination mechanism in Nasonia vitripennis (Hymenoptera; Chalcidoidea). Genetics 149:233-242.
Eickwort, G., et. al. 1996. Solitary behavior in a high-altitude population of the social sweat bee Halictus rubicundus. Behav. Ecol. Sociobiol.. 38: 227-233.
Godfray, H. 1994. Parasitoids: Behavioral and Evolutionary Ecology. Chapter 5. Princeton, NJ: Princeton University Press.
Hamilton, W., 1964. The genetical evolution of social behavior I.
J. Theoretical Biol. 7: 1-16.
Hardy, I., 1994. Sex ratio and mating structure in the parasitoid Hymenoptera. Oikos. 69: 3-20.
Hoffmann, A.A. and Turelli, M. 1997. Cytoplasmic incompatibility in insects. In Influentialpassengers -- inherited microorganisms and arthropod reproduction (ed. S.L.O'Neill, A.A. Hoffmann and J.H. Werren), Oxford University Press, pp. 42-80.
Hunt, G., and Page, R., 1995. Linkage map of the honey bee, Apis mellifera, based on RAPD markers. Genetics. 139: 1371-1382.
Marín, I., and Baker, B.S. 1998. The evolutionary dynamics of sex determination. Science. 281: 1990-1994.
Michener, C. 1974. The social behavior of the bees. Cambridge, Mass: Harvard University Press.
Packer, et. Al. 1989. The phenology and social biology of four sweat bees in a marginal environment: Cape Breton island. Canadian J. Zoology. 67: 2871-2877.
Periquet, G. et. al. 1993. Sex determination in the hymenopteran Diadronus pulchellus (Ichneumonidea): validation of the one-locus multi-allele model. Heredity, 70: 420-427.
Reed, K.M. and Werren, J.H. 1995. Induction of paternal genome loss by the paternal sex ratio chromosome and cytoplasmic incompatibility bacteria (Wolbachia): a comparative study of early embryonic events. Mol. Reprod. Dev. 40 (4): 408-418.
Ross, K, et. al. 1993. Effect of a founder event on variation in the genetic sex-determining system of the fire ant Solenopsis invicta. Genetics. 135: 843-854.
Ross, K., and Fletcher, D. 1986. Diploid male production- a significant colony mortality factor in the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol.. 19: 283-291.
Skinner, S.W. and Werren, J.H. 1980. The genetics of sex determination in Nasonia vitripennis (Hymenoptera: Pteromalidae). Genetics 94: s98.
Stouthamer, R. 1997. Wolbachia-induced pathenogenesis. In Influential passengers -- inherited microorganisms and arthropod reproduction (ed. S.L. O'Neill, A.A. Hoffmann and J.H. Werren), Oxford University Press, pp. 102-124.
Stouthamer, R. and Kazmer, D.J. 1994. Cytogenetics of microbe-associated parthogenesis and its consequences for gene flow in Trichogramma wasps. Heredity. 73: 317-327.
Stouthamer, R., Luck, R.F. and Hamilton, W.D. 1990a. Antibiotics cause parthenogenic Trichogramma to revert to sex. Proc. Natl. Acad. Sci. USA 87: 2424-2427.
Stouthamer, R., Pinto, J.D., Platner, G.R., and Luck, R.F. 1990b. Taxonomic status of thelytokous forms of Trichogramma. Ann. Entomol. Soc. Am. 83: 475-581.
Wenseleers, T., Ito, F., Van Borm, S. Huybrechts, R., Volkaert, F. and Billen, J. 1998. Widespread occurence of the microorganism Wolbachia in ants. Proc. Royal Soc. London, Series B. 265: 1447-1452
Werren, J.H. 1997. Biology of Wolbachia. Ann. Rev. Entomol. 42:587-609.
Whiting, P. 1943. Multiple alleles in complementary sex determination of Habrabracon. Genetics. 28: 365-382.